‘Solid State’ Chemistry in Titan Ice Particles
| PIA Number | PIA20715 |
|---|---|
| Language |
|
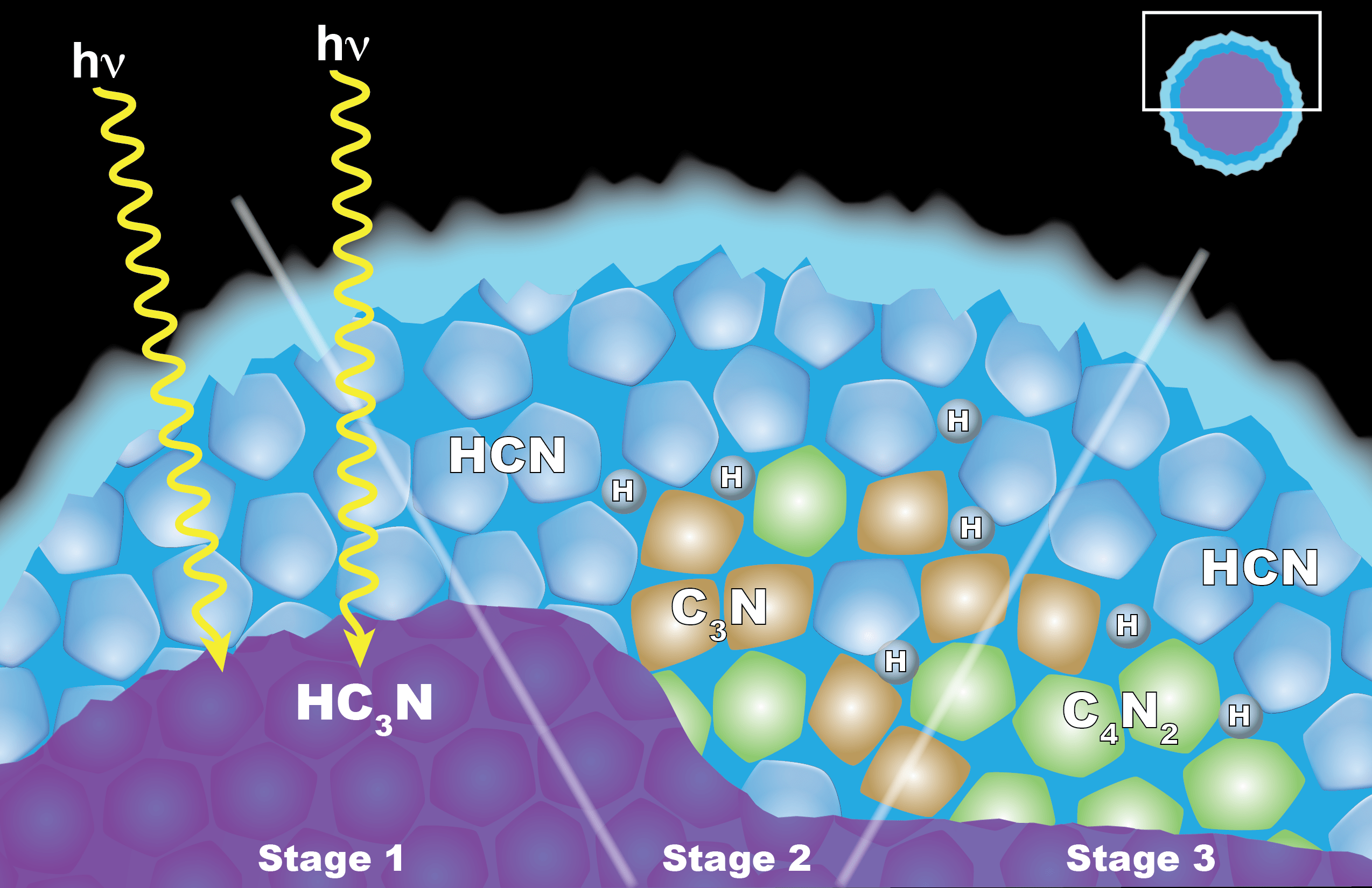
Scientists from NASA's Cassini mission suggested in a 2016 paper that the appearance of a cloud of dicyanoacetylene (C4N2) ice in Titan's stratosphere may be explained by “solid-state” chemistry taking place inside ice particles. The particles have an inner layer of cyanoacetylene (HC3N) ice coated with an outer layer of hydrogen cyanide (HCN) ice. Left: When a photon of light penetrates the outer shell, it can interact with the HC3N, producing C3N and H. Center: The C3N then reacts with HCN to yield C4N2 and H (shown at right). Another reaction that also yields C4N2 ice and H also is possible, but the researchers think it is less likely.
For a related news feature story, see: /news/12941/nasa-scientists-find-impossible-cloud-on-titan-again/ .
The Cassini-Huygens mission is a cooperative project of NASA, the European Space Agency and the Italian Space Agency. The Jet Propulsion Laboratory, a division of Caltech in Pasadena, manages the mission for NASA's Science Mission Directorate, Washington, D.C. The Cassini orbiter was designed, developed and assembled at JPL. The composite infrared spectrometer team is based at NASA's Goddard Space Flight Center, Greenbelt, Maryland, where the instrument was built.
For more information about the Cassini-Huygens mission visit http://www.nasa.gov/cassini and http://saturn.jpl.nasa.gov.
Image credit: NASA/JPL-Caltech/GSFC
